Drug recalls are important to stay on top and make sure you have all the latest details about, since, for one thing, most of us have medicine cabinets at home stocked with multiple over-the-counter therapeutics.
Moreover, agencies like the FDA issue recalls for items like these on a pretty regular basis. Because of everything from manufacturing problems that could include mislabeling a product, to the presence of a bacteria that was found, or some other problem. Examples include the previous drug recalls we’ve written about, such as two that involve potential contamination with microbes. They are the Senna Syrup recall and the Rompe Pecho recall. Below, meanwhile, are details of two more to know about.
Geri-Care aspirin and acetaminophen drug recall
First up is a drug recall action from Geri-Care Pharmaceuticals that involves bottles of aspirin and acetaminophen. Those are, of course, two of the most common drugs that people keep stored in their medicine cabinets.
For many people, in fact, taking these pills might be the first course of action whenever they want to alleviate pain or reduce a fever. Unfortunately, this particular recall poses a risk of poisoning to children, who might find it unacceptably easy to get their hands on these medicines.
Along those lines, the recall here focuses on the requirement that these drugs must be stored in child-resistant packaging. And according to a press release from the US Consumer Product Safety Commission, that’s not the case with these Geri-Care bottles. Instead, children who get their hands on these bottles might be able to open them and swallow the contents.
As a result, Geri-Care is recalling about 800 units of aspirin and acetaminophen bottles. The bottles include anywhere between 250 and 1,000 tablets.
Additional details
The recall includes the following list that consumers will want to be aware of:
- Extra Strength Acetaminophen 500mg Tablets – 1,000 tablets
- Regular Strength Enteric Coated Aspirin 325mg Tablets – 250 tablets
- Regular Strength Enteric Coated Aspirin 325mg Tablets – 1,000 tablets
- Adult Low Dose Enteric Coated Aspirin 81mg Tablets – 300 tablets
- Adult Low Dose Enteric Coated Aspirin 81mg Tablets – 1,000 tablets
Geri-Care sold the aspirin and acetaminophen products online through August 2021. Also of note, they cost between $2 and $10.
These are the online stores where someone could have purchased these bottles from, in addition to Amazon: simplymedical.com, drugsupplystore.com, heypharma.com, otcsuperstore.com, blowoutmedical.com, vitamincoveusa.com, simplymedical.com, silverrodrx.com, zoro.com, healthproductsforyou.com, earthturns.com, cleanitsupply.com, herbspro.com, stomabags.com, ebay.com, atcmedical.com, and bettymillls.com.
RevitaDerm Wound Care Gel recall
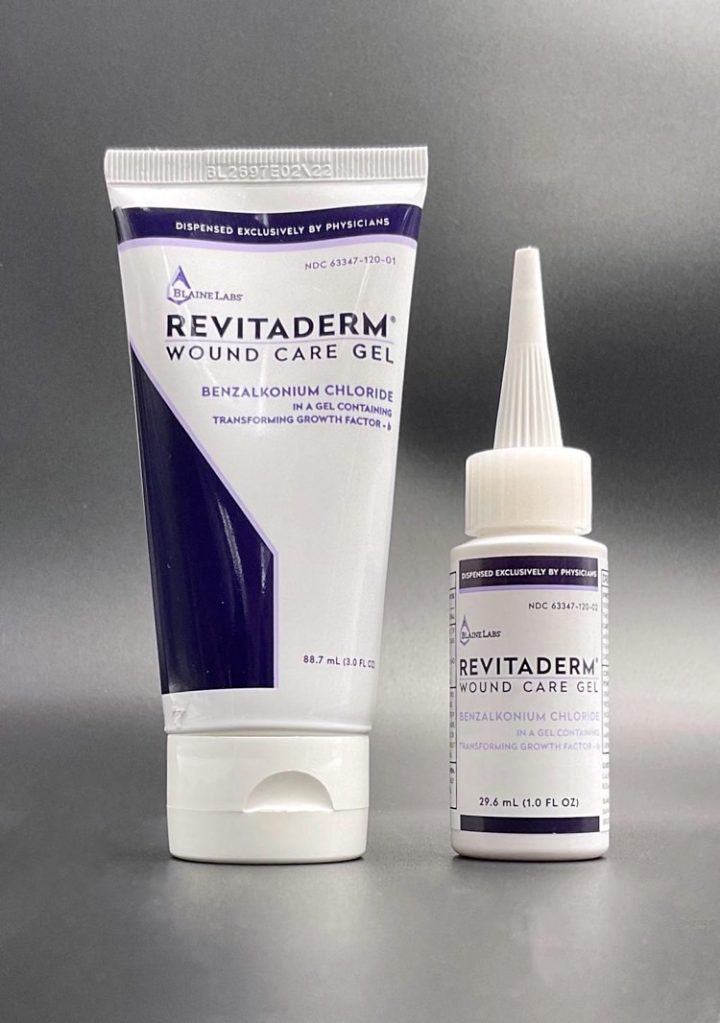
Another drug recall to highlight is one from Blaine Labs Company, which recently announced a wound care gel recall in conjunction with the US Food and Drug Administration (FDA).
The recall in question involves one lot of Revitaderm Wound Care Gel. And the company initiated the recall after discovering the presence of a bacteria (Bacillus cereus) in a bottle from that lot.
Revitaderm Wound Care Gel is a drug you can use as an antimicrobial on cuts and scrapes. The product comes in 1.0-ounce bottles and 3.0-ounce tubes, and the company distributed the product to 61 doctors in 17 states last year.
- Recall details: You should be looking for “BL 2844” on the package. And the expiration date is February 19, 2023. Which means the product has a long shelf life and could still be in homes around the country.
The announcement for this wound care gel recall explains that patients who use the product risk developing an infection with the Bacillus cereus bacteria. People who aren’t immunocompromised can expect less severe infections that typically respond to treatment. But the Bacillus cereus can be more dangerous to immunocompromised patients and preterm babies.
The bacteria can cause “life-threatening, invasive infections including wound and blood infections, sepsis, pneumonia, and meningitis.”