Aspirin, ibuprofen, and acetaminophen are common over-the-counter drugs that most people have around the house. The drugs can relieve pain and reduce fevers, among other things. Additionally, many people use these drugs to treat various other chronic health issues. But buyers should know there are four separate recall actions concerning various brands of aspirin, ibuprofen, and acetaminophen.
In total, we’re looking at around 400,000 bottles of these over-the-counter medicines that pose a poisoning risk, according to the US Consumer Product Safety Commission (CPSC). That’s because the drugs come in containers that are not child-resistant and thus do not meet the requirements of the Poison Prevention Packaging Act.
Aspirin, ibuprofen, and acetaminophen recall
The CPSC posted four separate recall notices on its website on June 16th. They cover aspirin, ibuprofen, and acetaminophen from various brands that violate the Poison Prevention Packaging Act (PPPA).
Substances including aspirin, ibuprofen, and acetaminophen must be packaged in child-resistant containers, according to the PPPA regulation. But the CPSC warns that around 400,000 bottles containing these pain relievers do not respect the rules. As a result, children might easily open the bottles and consume the drugs. In turn, this can lead to poisoning.
In what follows, we’ll discuss each of the different pain med recalls so you can quickly identify the faulty bottles.
Time-Cap aspirin and ibuprofen recall
Time-Cap Labs recalled 300-count Kroger aspirin and 160-count ibuprofen bottles that pose a risk of poisoning. The products were available from various retailers nationwide from July 2021 through March 2022.
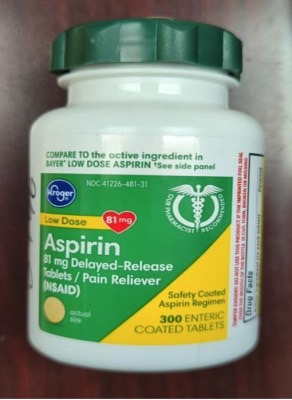
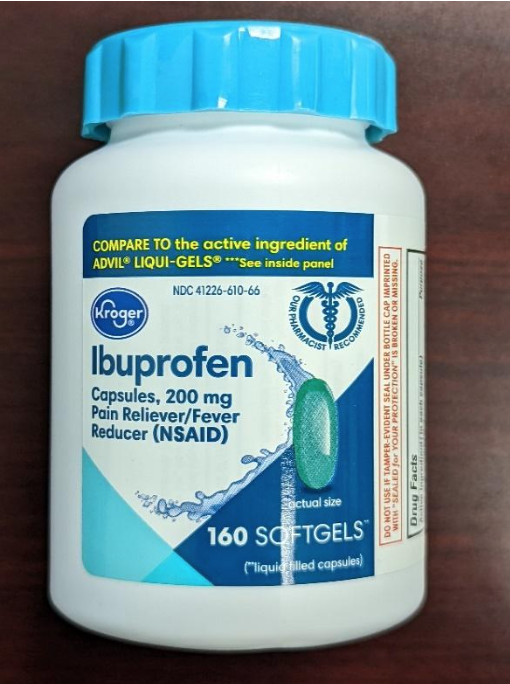
Kroger recalled 209,430 bottles with the following identifiers:
- Aspirin – UPC: 0004126001295; Lot numbers: A077J, F032H, F035H, J011H, K031H
- Ibuprofen – UPC: 0004126001298 Lot numbers: FH1163, C11044, C11047, C11064, C11065, C11079, C11084
The aspirin and ibuprofen recall announcement is available at this link.
Aurohealth acetaminophen recalls
Also, two types of Aurohealth acetaminophen were hit with a recall.
Aurohealth recalled about 25,660 bottles of 225-count Kroger-brand acetaminophen that fail to meet PPPA regulations. The Kroger-branded acetaminophen was available from December 2021 through March 2022 from various retailers nationwide. You can see the press release at this link.
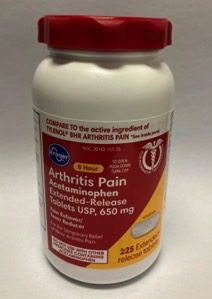
Look for the following identifiers for this acetaminophen recall:
- UPC number 0004126001284 and lot numbers P2100890, P2100891, P2100992 (each with expiration date Aug-2023) and P2101010 (with expiration date Apr-2023)
Additionally, Aurohealth recalled about 137,300 bottles of 150-count Walgreens-branded acetaminophen bottles. The drugs were available from Walgreens stores nationwide between October 2021 and April 2022. That announcement is available at this link.
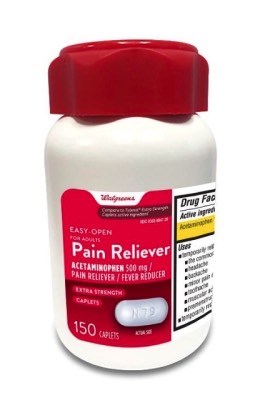
Look for the following identifiers on the bottles in your medicine cabinet:
- UPC number 311917218090 and Lot numbers P2100627, P2100671, P2100672, P2100689 P2100747, P2100859 (each with expiration date Nov-2022) and P2200050 (with expiration date Jan-2023)
Sun Pharma acetaminophen recall
Finally, Sun Pharma issued a recall for about 34,660 bottles of 100-count Kroger Acetaminophen that can poison children. The drug was available nationwide at multiple retailers from October 2021 through March 2022.

Look for these identifiers to determine whether or not the drug packaging is child-resistant. Any bottles with the following UPC number and lot codes are part of the recall:
- UPC number is 0004126001287 with the batch codes AC45463, AC38213 or AC30682
You’ll find the recall press release is available at the CPSC.
What you should do
We saw similar recalls earlier this year, as various drugs failed to meet the PPPA regulations.
If you own any of the aspirin, ibuprofen, and acetaminophen products from the new recalls, you should make sure you store the containers out of the reach of children. You can still use the drugs, as there’s nothing wrong with the chemical compounds. They only pose a risk of poisoning to young children. Older kids and adults should know how to use the drugs properly.
You can still keep the pills if you don’t have any kids in your household. But the drug companies behind these recalls advise buyers to dispose of the products or return them to receive a full refund.